Theme:
Regulatoryaffairs-2020
Conference Series Ltd conferences, "9th International Conference on Pharmaceutical restrictive Affairs and IPR" throughout Oct 16-17, 2020 in Munich,Germany. European nation aims to collect leading educational scientists, researchers, specialists and analysis students to exchange and share their experiences and analysis results on all aspects of Pharmaceutical restrictive Affairs. It together provides a premier content platform for researchers, practitioners, and educators to gift and discuss trends, the foremost recent innovations, and considerations yet as sensible challenges encountered and solutions adopted within the fields of Pharmaceutical restrictive Affairs and IPR.
REGULATORY AFFAIRS-2020 aims square measure to have interaction with new audiences to participate in activities on the day, Of course, we tend to conjointly need to achieve existing followers of Pharmaceutical social media accounts and to expand the world reach. Finally, this can be a good chance to explore as a replacement science communication tool. we tend to area unit presently checking out people to contribute, and to help us to create Pharma ‘go viral’!
We warmly welcome all the eminent researchers, delegates, students and to require part during this upcoming REGULATORYAFFAIRS event, to witness invaluable scientific discussions and contribute to the longer term advancements in the field of Pharmaceutical Sciences and its allied areas. We also feature exhibitions, workshops, and student research competitions that develop networking opportunities for all attendees.
REGULATORYAFFAIRS introduces an exceptional event to meet the Editorial Board Members and various masters in the Pharmaceutical Sciences field everywhere throughout the world and listen to the best speakers on new research extension. REGULATORYAFFAIRS Conference focus on sharing exploration on Regulatory Affairs, Regulatory Strategies and developments Regulatory Affairs Regulatory Strategies and developments, Intellectual Property Rights ,Impact of Brexit on Regulatory Framework.
Why to attend?
Who Should Attend ?
-
Pharmacovigilance Students, Scientists
-
Pharmacovigilance Researchers & Teachers
-
Medical Colleges
-
Pharmaceutical Industries
-
Pharmacovigilance Associations and Societies
-
Pharmacovigilance Training Institutes
-
Software Developing Companies
-
Medical Devices Manufacturing Companies
-
Data Management Companies
-
Business Entrepreneurs
Also, Directors/Senior Directors/Executive Directors and Vice Presidents/Senior Vice Presidents/Executive Vice Presidents and Heads/Leaders/Partners of:
-
CROs and CMOs
-
Clinical Research Sites
-
Pharma/Biotech and Medical Device industries
-
Hospitals, Associations
University Faculties scientists who are related to clinical and medical research like
-
Directors
-
Senior Professors
-
Assistant Professors
-
Associate Professor
-
Research Scholars
-
Ph.D Students
Regulatory Affairs is a nearly new calling which created from the craving of governments to ensure general wellbeing by controlling the security and capability of items in zones including pharmaceuticals , veterinary meds, clinical gadgets, pesticides, agrochemicals, beauty care products and correlative drugs. Regulatory Affairs is engaged with the improvement of new therapeutic items from right off the bat, by coordinating administrative standards and by getting ready and presenting the important administrative dossiers to wellbeing specialists.
Regulatory Affairs is effectively associated with each phase of improvement of another medication and in the post-showcasing exercises with approved curative items. The Regulatory Affairs office is a significant piece of the hierarchical structure of pharmaceutical industry. Inside it communicates at the interphase of medication improvement, assembling, promoting and clinical research. Remotely it is the key interface between the organization and the administrative specialists.
Regulatory Communications and Submissions
Because of the developing enthusiasm for promoting new items, it is crucial to set up an unrivaled degree of clinical composition and administrative tasks. Officials inside these jobs need to make and oversee fruitful entries, just as streamline courses of events, to quicken confirmation.
Going to the Optimizing Regulatory Communications and Submissions gathering will help assigns in speeding up the authorization of medications while meeting fundamentals for various administrative offices around the world. Likewise, participants will defeat key operational difficulties affecting the endorsement procedure to improve the nature of entries.
Regulatory Challenges for Medical Devices
As restorative device quality confirmation and regulatory endeavors specialists, it can test to stay centered of changes event in our industry. Managerial controls for remedial contraptions are uncommon in the making scene, in spite of the way that utilization of national restorative device headings will every now and again address the very issues raised in countries as huge mindfulness toward accepting prosperity. Instance of these issues join the illegal re-getting ready and re-packaging of used syringes for re-bargain; the openness accessible of equipment that misfires least quality and security benchmarks; or simply no trace of what devices are being sold in the country, nor by whom. Such a presenting is essential on enable governments to give alerts or surveys for hazardous or deficient things.
Medical Devices and Combination Product Regulations
Composite items are healing and characteristic things that merge meds, devices, and additionally natural things. FDA wants to get extensive amounts of mix things for review as imaginative advances continue combining thing sorts and cloud the obvious lines of segment between FDA's remedial thing centre, which are included the Centre for Biologics Evaluation and Research (CBER), the Centre for Drug Evaluation and Research (CDER), and the Centre for Devices and Radiological Health (CDRH). Since blend things incorporate parts that would consistently be controlled under different sorts of regulatory forces, and a significant part of the time by different FDA Centre, they raise testing authoritative, technique, and review organization challenges. Differentiations in regulatory pathways for each part can influence the managerial methods for all pieces of thing improvement and organization, including preclinical testing, clinical assessment, displaying applications, gathering and quality control, adversarial event announcing, headway and advancing, and post-support adjustments.
Regulatory Affairs in Pharmacovigilance
Regulatory Affairs (RA), moreover called government issues, are a calling inside oversaw endeavors, for instance, pharmaceuticals, therapeutic machines, essentialness, setting aside cash, telecom, etc. Regulatory Affairs moreover has a specific significance inside the human administrations undertakings (pharmaceuticals, restorative devices, biologics and helpful sustenances). Managerial issues (helpful issues) specialists (also called regulatory specialists) commonly have obligation with respect to the going with general regions:
Working with government, state and neighbourhood regulatory workplaces and workforce on specific issues affecting their business i.e.,working with such a great amount of office as the Food and Drug Management or European Medicines Agency (pharmaceuticals and remedial devices); The Department of Energy; or the Securities and Exchange Commission (banking). Prompting their associations on the regulatory edges and environment that would impact proposed works out. i.e., depicting the "authoritative air" around issues, for instance, the headway of expertly recognized prescriptions and Sarbanes-Oxley consistence
Global Regulatory Intelligence
Ever-multiplying and changing worldwide guidelines produce complex difficulties of execution in worldwide market freedom activities. Strategic choices are deferred, catching new worldwide income until showcase flexibility process issues are settled. Work process is jumbled as data clashes from deals centered merchants, recently delegated outside controllers, and heritage assets are accommodated and as conclusive answers are looked for. Administration is undermined as data holes lessen confirmation levels and block hazard the board of freedom related punishments, fines, and legal expenses.Furthermore, the significant level of business execution required to make an encouragement from continuous medtech industry combination isn't possible, since worldwide market scope activities are unequipped for quickly finishing new market freedom entries for recently procured product offerings.Not at all like repackaged RI databases planned for the pharmaceutical business, the clinivation Worldview Enterprise Solution for On-Demand Global Regulatory Intelligence quickens the market scope cycle.
Regulatory Strategies and developments
An administrative system is a conventional collection that adjusts the administrative exercises to put up another or changed item for sale to the public with the business procedure for that item. It gives in general definition and heading to the task group for the item being created by recognizing the significant administrative components to be routed to showcase the gadget. Inside an organization, there are generally numerous players from an mixture of divisions who chip away at building up the administrative methodology. Since this technique includes all parts of a medication, people with ability in compound combination, toxicology, science, clinical and administrative issues, showcasing, government issues, and repayment should all give contribution to the administrative procedure to guarantee it is as far reaching as could be allowed.
The administrative system report has three principle purposes. It should be:
A following device to condense key understandings came to with wellbeing specialists
An arranging instrument that reports courses of events and records subjects to address in future gatherings with wellbeing specialists
A hazard register to record key issues that could affect courses of events, expenses or business esteem for the venture
A biopharmaceutical, in any case called a biologic remedial thing or biologic, is any helpful thing made in, removed from, or semi arranged from natural sources. Not exactly equivalent to artificially fused pharmaceuticals, they combine vaccinations, blood, or blood fragments, allergenic, considerable cells, quality medications, tissues, recombinant therapeutic protein, and living cells used as a piece of cell treatment. Biologics can be made out of sugars, proteins, or nucleic acids or complex blends of these substances, or may live cells or tissues. They are bound from ordinary sources—human, animal, or microorganism. Stating enveloping biopharmaceuticals changes among social occasions and components, with different terms insinuating different subsets of therapeutics inside the general biopharmaceutical class. Some authoritative workplaces use the terms characteristic helpful things or medicinal natural thing to insinuate especially to structured macromolecular things like protein-and nucleic corrosive based medications, remembering them from things like blood, blood sections, or antibodies, which are commonly removed explicitly from a characteristic source Gene-based and cell biologics, for example, much of the time are at the bleeding edge of biomedical research, and may be used to treat a variety of remedial conditions for which the same meds are open.
Penalties for Regulatory Non-compliance
Non-compliance costs will be costs which result from an inability to conform to guideline. These expenses are not viewed as a major aspect of the administrative weight for the reasons for the RBM. This includes punishments and related exercises that are required to be attempted because of revolution with a guideline. Administrative effects that emerge, up to the point that move is made to react to the doubt of a particular occasion of rebelliousness, are remembered for the RBM system. This includes hazard based systems that may focus on specific populaces without a particular occasion of doubt. "FCPIAA characterizes a CMP as any punishment, fine, or other approval that is for a particular money related sum as gave by Federal law; or has a most extreme sum accommodated by Federal law; and is evaluated or authorized by an office as per Federal law; and is measured or upheld compliant with an authoritative continuing or a common activity in the Federal Courts". Inability to agree to the law can prompt authorization activity including at least one of the accompanying: on-the-spot fines, arraignment, which conveys a greatest fine of $12,110, disciplinary activity, going from fines to retraction of the permit.
The Quality control in regular nourishment preparing has a critical job in expecting a great, protected and nutritious nourishment supply for the general population, for their great wellbeing and for the financial advantages got from exchange of protected and top notch nourishment. Quality control gathering additionally assumes a significant job in nutrition industry. Risk Analysis and Critical Control Points (HACCP) is an administration framework where sanitation is tended to through the investigation and control of natural, mixture, and physical dangers from crude material creation, acquirement and taking care of, to assembling, dispersion and utilization of the completed item. Nourishment, Drug, and Cosmetic Act is a lot of laws offering position to the US-FDA to administer the sanitation, wellbeing of medications, and beautifying agents. Cleaning, sanitization and cleanliness ought to be carefully kept up in nutrition industry.
Good Clinical Practices & Good Laboratory Practices
The role of ICH in planning GCP standards is to give a moral treatment to the subjects who are associated with the clinical preliminaries. Gathering on Good Clinical Practices is significant in light of the fact that it is a universal moral and logical quality standard for structuring, directing, recording and announcing preliminaries that include the interest of human subjects. The GLP Principles show fundamentals for and give general direction on the lead of all nonclinical wellbeing and ecological security examines, including invitro considers. Pre-clinical initiations survey the danger of a medication and inspect its latent capacity significances for the human body. These preliminaries are led in-vitro which is required to continue with clinical preliminaries. Great research facility practices ought to be followed for these pre-clinical preliminaries.
Pharmaceutical Technology is the guideline of drug store that allocate with the way toward turning an Active Pharmaceutical Ingredient (API) into a medicine to be utilized by patients. It is likewise an information base to drug store, pharmacology and furthermore pharmaceutical business. It depicts plans, strategies, instrumentation in the arrangement, producing, intensifying, bundling, apportioning, aggregating of opiate and different arrangements utilized in determinative and symptomatic e methods in the treatment of patients. It is also connected with the study of portion type.
Pharmaceutical consideration is characterized as the dependable arrangement of medication treatment to accomplish positive restorative results that improves the patients personal satisfaction. It includes the procedure through which a drug specialist helps out patients and different experts in planning, actualizing and checking a remedial arrangement will deliver explicit restorative results for the patients.
Recent trends in Pharma and Technology
Each industry is changing at an all the more quick pace. Items and organizations are finding a workable pace popularized. Development is having a constantly more noteworthy impact in ask about and improvement, displaying and advancing, and arrangements and scattering. The pharmaceutical division is one that has felt this change in research and progression in the course of recent years and will start to feel it inside different divisions over the one more decade. Almost certainly, inside the following decade, individuals will now not be pharmaceutical guineas pigs. Rather, intellectual PCs will be used in biotechnology and genomic look into. Rather than it taking a very long time to see the effect of a particular medication on a great many people, it'll take seconds to see the effect of thousands of medications on billions of recreations of the human body's physiology. Body sensors are another development that is correct currently still in clinical preliminaries. These sensors can either be put on the body or inside of it. They degree distinctive fundamental essential signs. With the unused development and progression, patients are getting more control. They by and by have the ability to have to a greater degree a state with regards to their body and their wellbeing. Right now, organizations should be beginning taking patient needs, musings, and wants into thought. Items will be increasingly compelling if patients are remembered for their creation and conveyance. Almost certainly, over the coming quite a while, various organizations inside the pharmaceutical business will make determined admonitory sheets. The pharmaceutical business is changing at each an each level.
Impact of Brexit on Regulatory Framework
The most significant bit of UK enactment that would should be canceled is the European Communities Act 1972 (ECA), which accommodates the matchless quality of EU law. Canceling the ECA will stop the protected relationship that exists among EU and UK law. In addition, the tremendous measures of auxiliary performing that have been passed with the goal and legitimization of executing EU law would need to be considered by the Government. EU Regulations depend on the standard of direct materialness, which implies that not at all like EU Directives, they are straightforwardly executed into UK law without the requirement for enactment from the UK Parliament. Right now, are all the more remarkable authoritative instruments for the EU due to their prompt relevance. The status of existing Regulations will be tended to in the Great Repeal Bill, in spite of the fact that as noted above, as a rule changes will probably be expected to consider the UK's new relationship with the EU. The Court of Justice of the European Union (CJEU) arranged in Luxembourg is the last authority on inquiries of the translation of EU law. In her first discourse setting out the UK Government's needs for Brexit on 17 January 2017, the Prime Minister rehashed her position that the UK isn't set up to keep on being dependent upon the ward of the CJEU.
Medication business is one in everything about country's most basic fiscal engines, exchange $15 billion in stock yearly and several its preparing plants area unit overwhelming. The administration office has developed a movement of tips for making GXP (which joins GCP, GLP and GMP bearings) that shield each the existence sciences business moreover the customers they serve. So as to help FDA-coordinated firms, master control offers accomplice in joined quality and consistence organization code that ensures GLP, GCP and GMP rules consistence. Industry rehearses is moreover some part of significant worth peril organization structure, Pharma Regulatory Affairs, Audits and evaluations, acknowledgment techniques (process endorsement and informative endorsement), Qualification, acknowledgment, alteration, upkeep, danger examination Drug Safety and Good Pharmacovigilance sharpens. A portion of our item system applications which will energize with GLP GCP GMP rules encapsulate the subsequent game plans: Document Control/Document Management, Corrective Action Preventive Action (CAPA), modification organization, controlling Management, opposition Automation programming structure, Audit Management in adventure with GLP GCP GMP rules, client fights programming system, shapes based methodology robotization, electronic passages.
Regulatory Affairs 2018
The “8th International Conference and Exhibition on Pharmaceutical Regulatory Affairs and IPR” (Regulatory Affairs 2018) hosted by Conference series LLC Ltd during June 08-09, 2018 at Philadelphia, Pennsylvania, USA with a theme “Regulatory considerations in pharmaceutical & biopharmaceutical research and market” was a great success where eminent Keynote Speakers from various reputed institutions with their presence addressed the gathering to explore ideas for reducing pollution.
Active participation and generous response was received from the Organizing Committee Members, Editorial Board Members of Journals as well as from Eminent Scientists, specialists, Clinicians, Professors, Talented Researchers, Speakers and Young Student Community.
The two day program witnessed thought provoking keynote and plenary presentations from experts in the field of Pharmaceutical Science, highlighting the theme,“Regulatory considerations in pharmaceutical & biopharmaceutical research and market”.which has encrusted the below scientific sessions:
- Regulatory Affairs
- Regulatory Communications and Submissions
- Regulatory Challenges for Medical Devices
- Medical Devices and Combination Product Regulations
- Regulatory Affairs in Pharmacovigilance
- Global Regulatory Intelligence
- Regulatory Strategies and developments
- Biologics and Biosimilars
- Penalties for Regulatory Non-compliance
- GMP in Food Industry
- Good Clinical Practices & Good Laboratory Practices
- Pharmaceutical Technology
- Pharmaceutical Care
- Recent trends in Pharma and Technology
- Impact of Brexit on Regulatory Framework
- Best Industry Practices
Regulatory Affairs 2018 Organizing Committee would like to thank the Moderator of the conference who contributed a lot for the smooth functioning of this event.
We would like to specially thank our Keynote Speakers & Speakers who participated avidly and effectively
- OumKaltoum Lahlou, University of Barcelona, Spain
- Aysu Yurdasiper Erdem, Ege University, Turkey
- Jagessar R C , University of Guyana, Guyana
Conference Series LLC Ltd communicates its appreciation to the Organizing Committee Members, Chair and Co-seat, Editorial Board Members of Conference Series LLC Ltd diaries, Speakers, Students, Sponsors, Exhibitors, famous characters and Media Partners in making Regulatory Affairs 2018 an extraordinary achievement. With the one of a kind input from the gathering, Conference Series LLC Ltd might want to report the beginning of the “8th International Conference and Exhibition on Pharmaceutical Regulatory Affairs and IPR” is going to be held during June 08-09, 2018 at Philadelphia, Pennsylvania, USA
Mark your calendars for the upcoming conference; we are hoping to see you soon!!!!
Let us meet again @ Regulatory Affairs 2020.
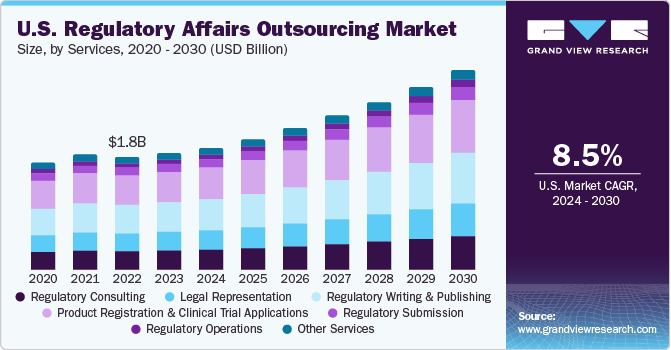
The worldwide administrative issues re-appropriating market size is required to reach USD 15.6 billion by 2027, growing at a CAGR of 11.9%, as indicated by another report by Grand View Research, Inc. The section of life sciences organizations in worldwide markets and the advancement of new territories, for example, vagrant medications, biosimilar, and customized medication are pushing market development.A noteworthy increment in the fixed expenses of in-house assets for medicinal services administrative issues and activity exercises like preparing, innovation, particular information, and offices are driving life science firms to redistribute these capacities. Additionally, the rising number of clinical preliminaries is relied upon to help the interest for the administrations, for example, clinical preliminary applications and item enrollment in these areas.
Item explicit clinical exhortation and procedure alongside human services administrative consistence at the beginning times of item improvement can be basic for the item endorsement. Inability to address the consistence in the early advancement organize frequently prompts delay in the endorsement procedure inferable from improperly structured examinations, fabricating oversights, excluded considers, and different disappointments to meet the administrative requirements.Tending to neighborhood administering difficulties and consistent changes in the guidelines of the significant markets, for example, U.S., Europe, and Asia is making interest for the administrations. Consistence with the present guidelines has become an massive task, take off alone attempting to remain current with advancements around the globe. Corrections to current guidelines are probably going to entangle the overseeing pathway for the business. This, thusly, is foreseen to drive the market for medicinal services administrative issues redistributing over the figure time frame.
Conference Highlights
- Regulatory Affairs
- Regulatory Communications and Submissions
- Regulatory Challenges for Medical Devices
- Medical Devices and Combination Product Regulations
- Regulatory Affairs in Pharmacovigilance
- Global Regulatory Intelligence
- Regulatory Strategies and developments
- Biologics and Biosimilars
- Penalties for Regulatory Non-compliance
- GMP in Food Industry
- Good Clinical Practices & Good Laboratory Practices
- Pharmaceutical Technology
- Pharmaceutical Care
- Recent trends in Pharma and Technology
- Impact of Brexit on Regulatory Framework
- Best Industry Practices
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | October 16-17, 2020 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | ||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Research & Reviews in Pharmacy and Pharmaceutical Sciences
- Clinical Pharmacology & Biopharmaceutics
- Research & Reviews: Journal of Pharmaceutical Analysis
Abstracts will be provided with Digital Object Identifier by




